Spectroscopic Methods on:
[Wikipedia]
[Google]
[Amazon]
Applied spectroscopy is the application of various
 Many polymers are attacked by
Many polymers are attacked by
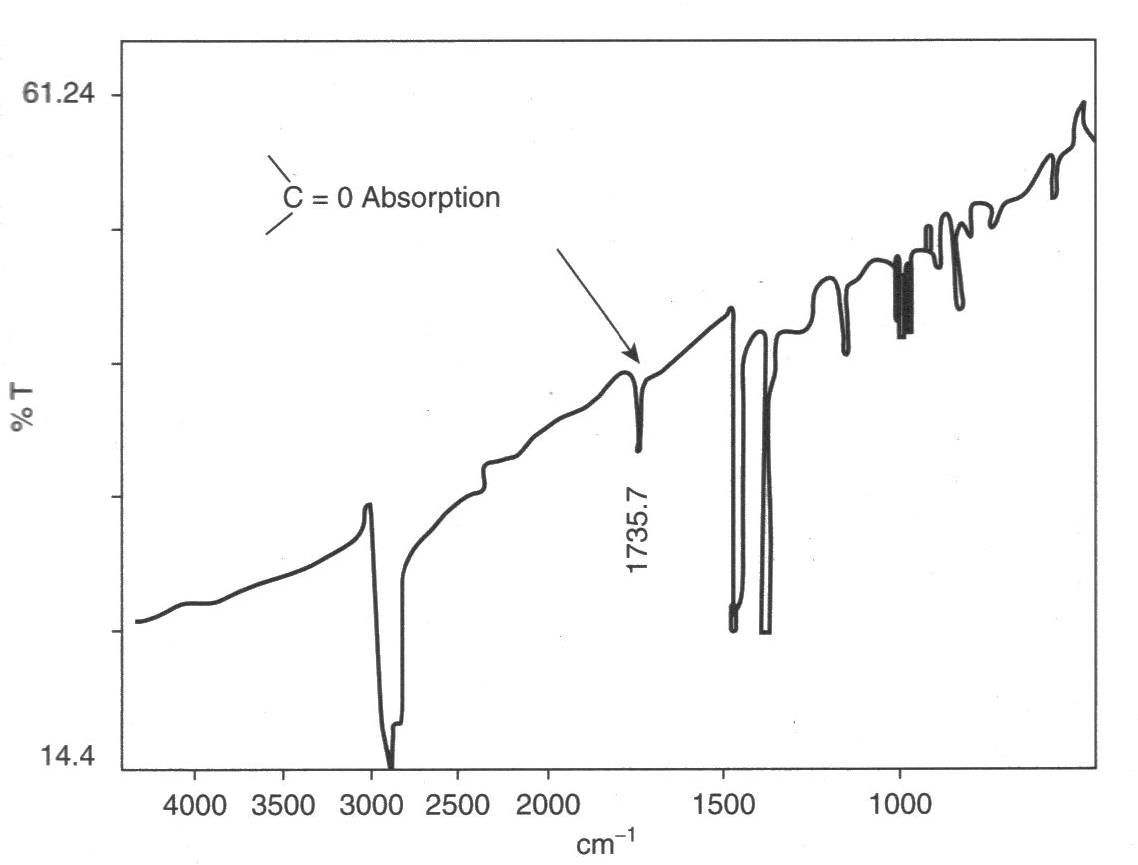 Polymers are susceptible to attack by atmospheric
Polymers are susceptible to attack by atmospheric

 The reaction occurring between double bonds and ozone is known as
The reaction occurring between double bonds and ozone is known as 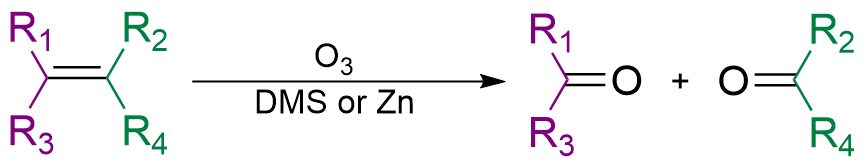 The immediate result is formation of an
The immediate result is formation of an
spectroscopic
Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter wav ...
methods for the detection and identification of different elements or compound
Compound may refer to:
Architecture and built environments
* Compound (enclosure), a cluster of buildings having a shared purpose, usually inside a fence or wall
** Compound (fortification), a version of the above fortified with defensive struct ...
s to solve problems in fields like forensics
Forensic science, also known as criminalistics, is the application of science to criminal and civil laws, mainly—on the criminal side—during criminal investigation, as governed by the legal standards of admissible evidence and crimina ...
, medicine
Medicine is the science and practice of caring for a patient, managing the diagnosis, prognosis, prevention, treatment, palliation of their injury or disease, and promoting their health. Medicine encompasses a variety of health care pract ...
, the oil industry
The petroleum industry, also known as the oil industry or the oil patch, includes the global processes of exploration, extraction, refining, transportation (often by oil tankers and pipelines), and marketing of petroleum products. The larges ...
, atmospheric chemistry
Atmospheric chemistry is a branch of atmospheric science in which the chemistry of the Earth's atmosphere and that of other planets is studied. It is a multidisciplinary approach of research and draws on environmental chemistry, physics, meteoro ...
, and pharmacology
Pharmacology is a branch of medicine, biology and pharmaceutical sciences concerned with drug or medication action, where a drug may be defined as any artificial, natural, or endogenous (from within the body) molecule which exerts a biochemica ...
.
Spectroscopic methods
A common spectroscopic method for analysis isFourier transform infrared spectroscopy
Fourier-transform infrared spectroscopy (FTIR) is a technique used to obtain an infrared spectrum of absorption or emission of a solid, liquid, or gas. An FTIR spectrometer simultaneously collects high-resolution spectral data over a wide spectr ...
(FTIR), where chemical bonds can be detected through their characteristic infrared absorption frequencies or wavelengths. These absorption characteristics make infrared analyzers an invaluable tool in geoscience, environmental science, and atmospheric science. For instance, atmospheric gas monitoring has been facilitated by the development of commercially available gas analyzers which can distinguish between carbon dioxide, methane, carbon monoxide, oxygen, and nitric oxide.
Ultraviolet (UV) spectroscopy is used where strong absorption of UV radiation
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation i ...
occurs in a substance. Such groups are known as chromophores
A chromophore is the part of a molecule responsible for its color.
The color that is seen by our eyes is the one not absorbed by the reflecting object within a certain wavelength spectrum of visible light. The chromophore is a region in the molec ...
and include aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
groups, conjugated system
In theoretical chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in a molecule, which in general lowers the overall energy of the molecule and increases stability. It is conventionally represented as ...
of bonds, carbonyl group
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a ...
s and so on. Nuclear magnetic resonance spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. The sample is placed in a magnetic fiel ...
detects hydrogen atoms in specific environments, and complements both infrared (IR) spectroscopy and UV spectroscopy. The use of Raman spectroscopy
Raman spectroscopy () (named after Indian physicist C. V. Raman) is a spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency modes of systems may also be observed. Raman sp ...
is growing for more specialist applications.
There are also derivative methods such as infrared microscopy
Microscopy is the technical field of using microscopes to view objects and areas of objects that cannot be seen with the naked eye (objects that are not within the resolution range of the normal eye). There are three well-known branches of micr ...
, which allows very small areas to be analyzed in an optical microscope
The optical microscope, also referred to as a light microscope, is a type of microscope that commonly uses visible light and a system of lenses to generate magnified images of small objects. Optical microscopes are the oldest design of microsco ...
.
One method of elemental analysis
Elemental analysis is a process where a sample of some material (e.g., soil, waste or drinking water, bodily fluids, minerals, chemical compounds) is analyzed for its elemental and sometimes isotopic composition. Elemental analysis can be qualita ...
that is important in forensic analysis
Forensic science, also known as criminalistics, is the application of science to criminal and civil laws, mainly—on the criminal side—during criminal investigation, as governed by the legal standards of admissible evidence and criminal p ...
is energy-dispersive X-ray spectroscopy
Energy-dispersive X-ray spectroscopy (EDS, EDX, EDXS or XEDS), sometimes called energy dispersive X-ray analysis (EDXA or EDAX) or energy dispersive X-ray microanalysis (EDXMA), is an analytical technique used for the elemental analysis or chemi ...
(EDX) performed in the environmental scanning electron microscope
The environmental scanning electron microscope (ESEM) is a scanning electron microscope (SEM) that allows for the option of collecting electron micrographs of specimens that are wet, uncoated, or both by allowing for a gaseous environment in ...
(ESEM). The method involves analysis of back-scattered X-rays from the sample as a result of interaction with the electron beam. Automated EDX is further used in a range of automated mineralogy Automated mineralogy is a generic term describing a range of analytical solutions, areas of commercial enterprise, and a growing field of scientific research and engineering applications involving largely automated and quantitative analysis of mine ...
techniques for identification and textural mapping.
Sample preparation
In all three spectroscopic methods, the sample usually needs to be present in solution, which may present problems during forensic examination because it necessarily involves sampling solid from the object to be examined. In FTIR, three types of samples can be analyzed: solution ( KBr), powder, or film. A solid film is the easiest and most straight forward sample type to test.Analysis of polymers
Manypolymer degradation
Polymer degradation is the reduction in the physical properties of a polymer, such as strength, caused by changes in its chemical composition. Polymers and particularly plastics are subject to degradation at all stages of their product life cycle ...
mechanisms can be followed using IR spectroscopy, such as UV degradation
In polymer chemistry photo-oxidation (sometimes: oxidative photodegradation) is the degradation of a polymer surface due to the combined action of light and oxygen. It is the most significant factor in the weathering of plastics. Photo-oxidatio ...
and oxidation, among many other failure modes.
UV degradation
 Many polymers are attacked by
Many polymers are attacked by UV radiation
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation i ...
at vulnerable points in their chain structures. Thus, polypropylene
Polypropylene (PP), also known as polypropene, is a thermoplastic polymer used in a wide variety of applications. It is produced via chain-growth polymerization from the monomer propylene.
Polypropylene
belongs to the group of polyolefins and ...
suffers severe cracking in sunlight unless anti-oxidant
Antioxidants are compounds that inhibit oxidation, a chemical reaction that can produce free radicals. This can lead to polymerization and other chain reactions. They are frequently added to industrial products, such as fuels and lubricants, ...
s are added. The point of attack occurs at the tertiary carbon atom
Tertiary ( ) is a widely used but obsolete term for the geologic period from 66 million to 2.6 million years ago.
The period began with the demise of the non- avian dinosaurs in the Cretaceous–Paleogene extinction event, at the start ...
present in every repeat unit, causing oxidation and finally chain breakage. Polyethylene
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging ( plastic bags, plastic films, geomembranes and containers including bo ...
is also susceptible to UV degradation, especially those variants that are branched polymers such as low-density polyethylene
Low-density polyethylene (LDPE) is a thermoplastic made from the monomer ethylene. It was the first grade of polyethylene, produced in 1933 by Imperial Chemical Industries (ICI) using a high pressure process via free radical polymerization. Its ...
. The branch points are tertiary carbon atoms, so polymer degradation
Polymer degradation is the reduction in the physical properties of a polymer, such as strength, caused by changes in its chemical composition. Polymers and particularly plastics are subject to degradation at all stages of their product life cycle ...
starts there and results in chain cleavage, and embrittlement. In the example shown at left, carbonyl groups were readily detected by IR spectroscopy from a cast thin film. The product was a road cone that had cracked in service, and many similar cones also failed because an anti-UV additive had not been used.
Oxidation
 Polymers are susceptible to attack by atmospheric
Polymers are susceptible to attack by atmospheric oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
, especially at elevated temperatures encountered during processing to shape. Many process methods such as extrusion
Extrusion is a process used to create objects of a fixed cross-sectional profile by pushing material through a die of the desired cross-section. Its two main advantages over other manufacturing processes are its ability to create very complex c ...
and injection moulding
Injection moulding (U.S. spelling: injection molding) is a manufacturing process for producing parts by injecting molten material into a mould, or mold. Injection moulding can be performed with a host of materials mainly including metals (for ...
involve pumping molten polymer into tools, and the high temperatures needed for melting may result in oxidation unless precautions are taken. For example, a forearm crutch suddenly snapped and the user was severely injured in the resulting fall. The crutch had fractured across a polypropylene insert within the aluminium tube of the device, and IR spectroscopy of the material showed that it had oxidised, possibly as a result of poor moulding.
Oxidation is usually relatively easy to detect, owing to the strong absorption by the carbonyl group in the spectrum of polyolefins
A polyolefin is a type of polymer with the general formula (CH2CHR)n where R is an alkyl group. They are usually derived from a small set of simple olefins (alkenes). Dominant in a commercial sense are polyethylene and polypropylene. More speciali ...
. Polypropylene has a relatively simple spectrum, with few peaks at the carbonyl position (like polyethylene). Oxidation tends to start at tertiary carbon atoms because free radicals
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron.
With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spont ...
here are more stable, so last longer and are attacked by oxygen. The carbonyl group can be further oxidised to break the chain, so weakening the material by lowering the molecular weight
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
, and cracks start to grow in the regions affected.
Ozonolysis

 The reaction occurring between double bonds and ozone is known as
The reaction occurring between double bonds and ozone is known as ozonolysis
In organic chemistry, ozonolysis is an organic reaction where the unsaturated bonds of alkenes (), alkynes (), or azo compounds () are cleaved with ozone (). Alkenes and alkynes form organic compounds in which the multiple carbon–carbon bond ...
when one molecule of the gas reacts with the double bond:
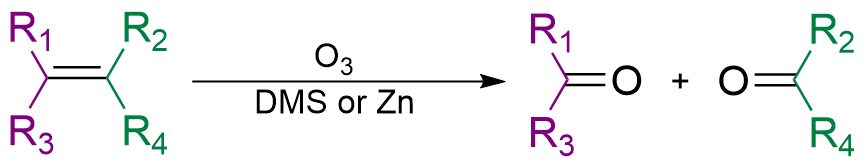 The immediate result is formation of an
The immediate result is formation of an ozonide
Ozonide is the polyatomic anion . Cyclic organic compounds formed by the addition of ozone () to an alkene are also called ozonides.
Ionic ozonides
Inorganic ozonides are dark red salts. The anion has the bent shape of the ozone molecule.
Inor ...
, which then decomposes rapidly so that the double bond is cleaved. This is the critical step in chain breakage when polymers are attacked. The strength of polymers depends on the chain molecular weight or degree of polymerization
The degree of polymerization, or DP, is the number of monomeric units in a macromolecule or polymer or oligomer molecule.
For a homopolymer, there is only one type of monomeric unit and the ''number-average'' degree of polymerization is given by ...
: The higher the chain length the greater the mechanical strength (such as tensile strength
Ultimate tensile strength (UTS), often shortened to tensile strength (TS), ultimate strength, or F_\text within equations, is the maximum stress that a material can withstand while being stretched or pulled before breaking. In brittle materials t ...
). By cleaving the chain, the molecular weight drops rapidly and there comes a point when it has little strength whatsoever, and a crack forms. Further attack occurs in the freshly exposed crack surfaces and the crack grows steadily until it completes a circuit and the product separates or fails. In the case of a seal or a tube, failure occurs when the wall of the device is penetrated.
The carbonyl end groups that are formed are usually aldehydes
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group ...
or ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
s, which can oxidise further to carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
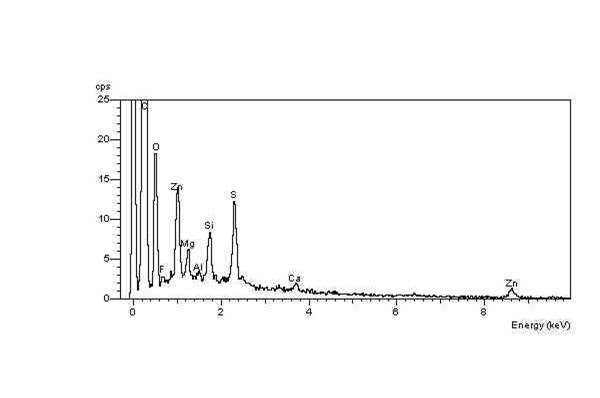
s. The net result is a high concentration of elemental oxygen on the crack surfaces, which can be detected using EDX in the ESEM. For example, two EDX spectra were obtained during an investigation into ozone cracking
Cracks can be formed in many different elastomers by ozone attack, and the characteristic form of attack of vulnerable rubbers is known as ozone cracking. The problem was formerly very common, especially in tires, but is now rarely seen in those ...
of diaphragm seal
A diaphragm seal is a flexible membrane that seals and isolates an enclosure. The flexible nature of this seal allows pressure effects to cross the barrier but not the material being contained.
Common uses for diaphragm seals are to protect pre ...
s in a semiconductor fabrication factory. The EDX spectrum of the crack surface shows the high-oxygen peak compared with a constant sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
peak. In contrast, the EDX spectrum of the unaffected elastomer surface spectrum shows a relatively low-oxygen peak compared with the sulfur peak.
See also
References
*''Forensic Materials Engineering: Case Studies'' by Peter Rhys Lewis, Colin Gagg, Ken Reynolds, CRC Press (2004). *Peter R Lewis and Sarah Hainsworth, ''Fuel Line Failure from stress corrosion cracking'', Engineering Failure Analysis,13 (2006) 946-962. *J. Workman and Art Springsteen (Eds.), ''Applied Spectroscopy: A Compact Reference for Practitioners'', Academic Press (1998) {{ISBN, 978-0-12-764070-9. Polymer chemistry Spectroscopy Analytical chemistry